A Level Chemistry is not just about memorizing the Periodic Table. It’s about pattern recognition and precision.
Most students fail because they try to rote-learn every single reaction (there are hundreds) or they panic in the lab exam. Top students don’t have better memories; they have better systems.
Need expert learning support? Check out our online tutoring
Student Pulse
The core struggles for 9701 students, evident in online forums and examiner reports, fall into three categories:
- Conceptual Gaps: Failing to understand the connection between topics like kinetics, equilibria, and energetics. Students treat them as isolated units (Source 1.1).
- Calculation Errors: Losing marks due to basic mathematical mistakes, incorrect use of significant figures, or failing to show required steps in quantitative questions (Source 1.2, 2.2).
- Wording/Precision: Missing marks on theory papers because definitions lack the specific key words required by the mark scheme (Source 1.5, 2.3).
These tips directly address the transition from content knowledge to applied exam technique.
Strategy Breakdown: 7 High-Impact Tips
Here are 7 Reddit-validated, examiner-backed strategies to master the 9701 syllabus.
1. The “Spider Map” Method (Organic Synthesis)
Rote-learning linear lists of reactions (e.g., “Alcohol + Acid $\rightarrow$ Ester”) fails when the exam asks for a 4-step synthesis.
- The Fix: Create a single A3 “Spider Map.”
- How: Put Alkenes in the center. Draw arrows out to every derivative (Alcohols, Haloalkanes, Polymers). Then draw arrows connecting those derivatives to each other.
It is difficult to memorize linear lists of reactions. To make this easier, here is a visual example of how to structure your ‘Spider Map’ radially:
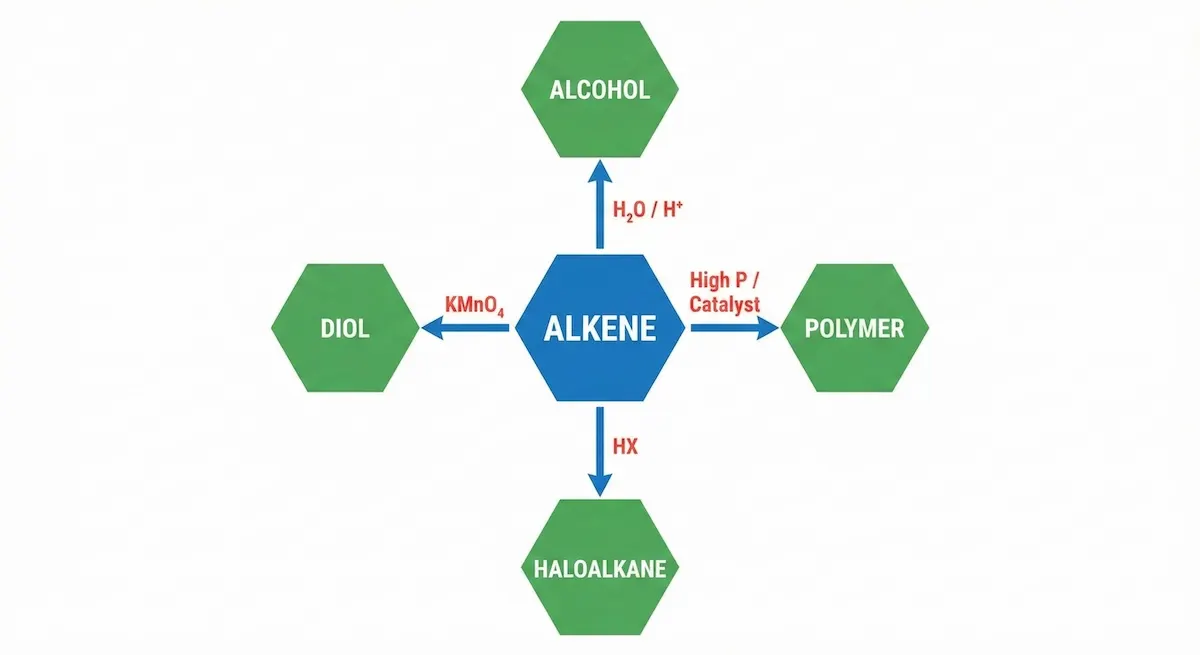
Stop rote-learning lists; use this Spider Map method to visualize all organic reaction pathways in one glance.
Notice how the Alkene sits in the center; in the exam, simply picture this shape to recall all potential derivatives at once.
- The Rule: Include Reagents and Conditions on every arrow. Use color codes: Red for Oxidation, Blue for Reduction, Green for Hydrolysis.
2. The “Titration Emergency” Protocol (Paper 3)
In the practical exam, your results might be terrible. Maybe you overshot the endpoint.
- The Secret: Accuracy marks are only ~4 marks out of 40. The rest are for calculation and consistency.
- The Fix: If you mess up a titration:
- Do not fake perfect results (examiners spot this).
- Instead, pick your two “closest” titres (even if they aren’t concordant).
- Tick them.
- Use the average of those ticked values for every subsequent calculation. You will lose the accuracy mark, but you will keep all 10+ calculation marks via “Error Carry Forward.”
In the high-pressure environment of Paper 3, panic is your enemy. Use the decision tree below to navigate a failed experiment without losing your calculation marks:
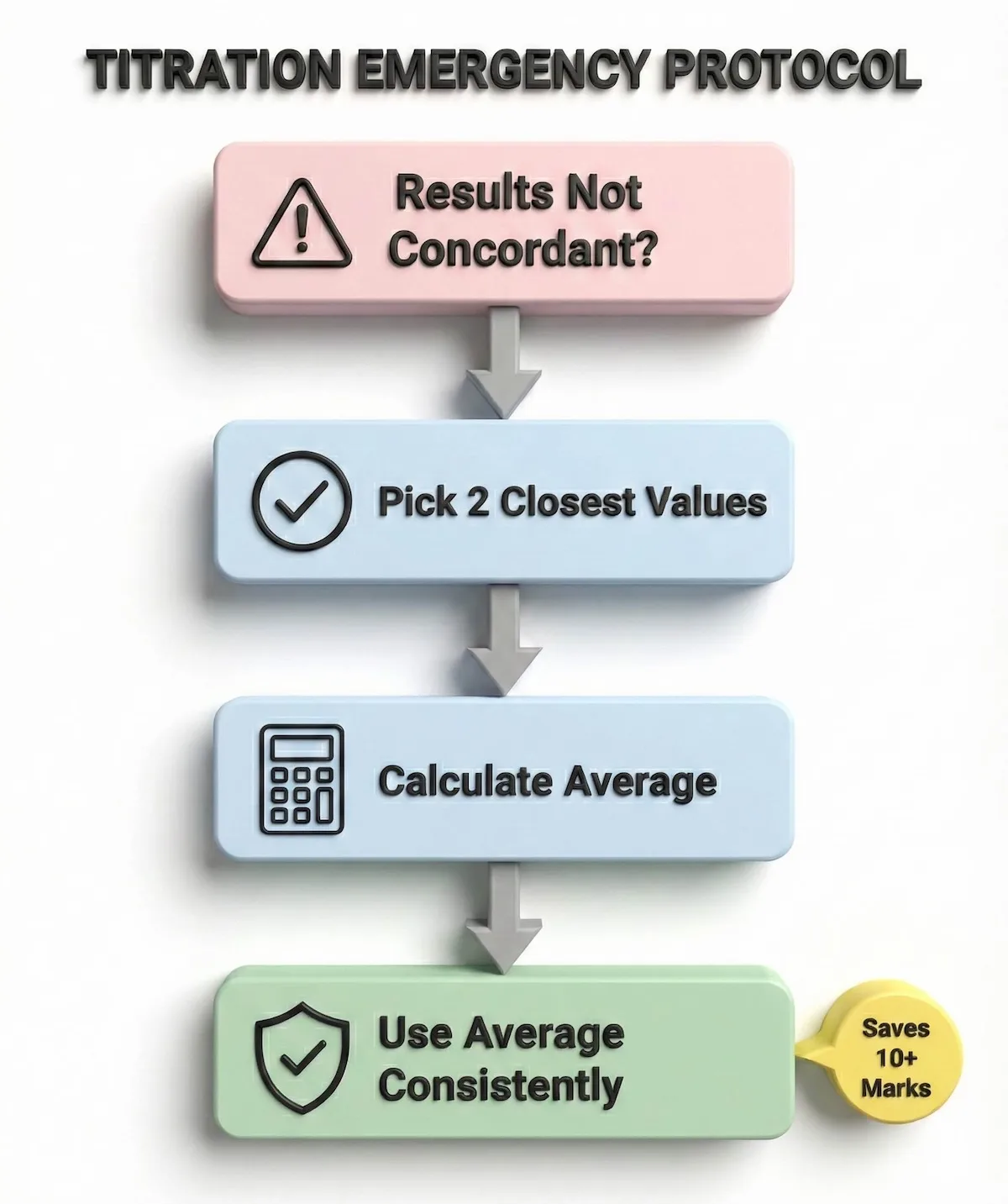
Don’t panic if your titration fails; follow this protocol to sacrifice accuracy marks but secure the calculation marks.
By following this protocol, you sacrifice the small accuracy marks to protect the much larger block of calculation marks.
Check out smart test prep solutions to score higher
3. Born-Haber “Sign & Multiply” Check
This is the most common calculation error in A Level Chemistry.
- The Trap: Calculating Lattice Energy for $MgCl_2$ or $Na_2O$.
- The Fix:
- Atomisation: Did you multiply $\Delta H_{at}$ by 2 for the two Chlorine atoms? ($Cl_2 \rightarrow 2Cl$)
- Affinity: Did you multiply Electron Affinity by 2?
- The Sign: Remember: Lattice Energy is formation from gaseous ions. If you are breaking a lattice, the sign flips.
Born-Haber cycles are calculation heavy and prone to sign errors. Before you finalize your answer, run through this visual checklist:
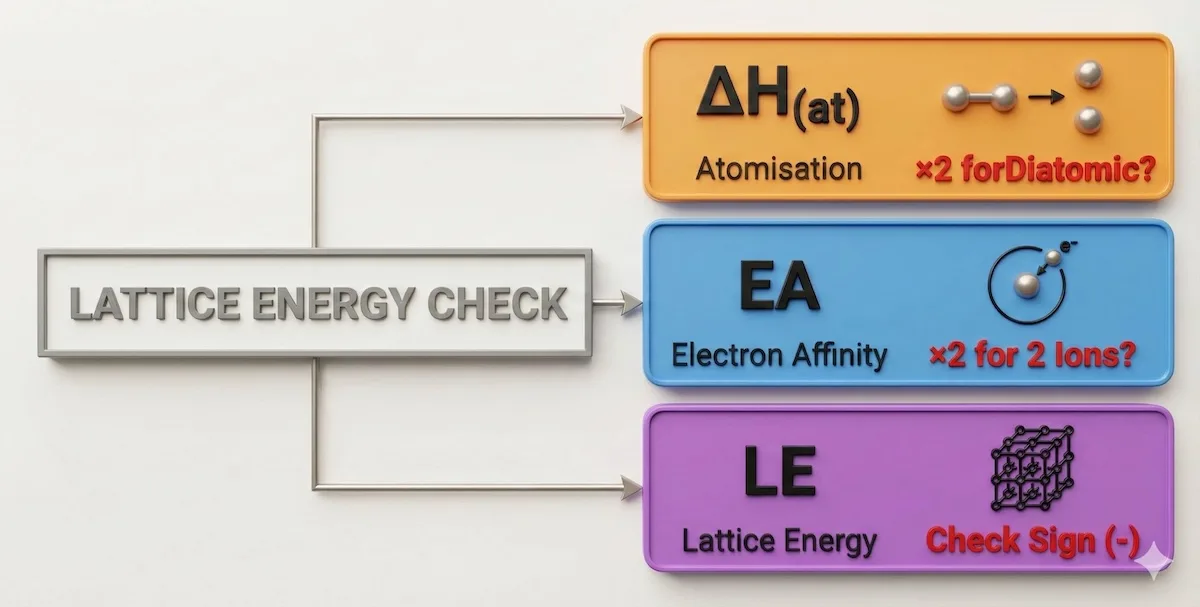
Use this “Sign & Multiply” checklist before finishing any Born-Haber calculation to catch the most common errors.
Missing that ‘×2’ multiplier for diatomic elements is the most common reason students lose marks here—don’t let that be you.
4. Transition Metal Mnemonics (Vanadium)
You need to know the colours of Vanadium oxidation states (+5, +4, +3, +2).
- The Mnemonic: “You Better Get Vanadium”
o Yellow (+5)
o Blue (+4)
o Green (+3)
o Violet (+2)
5. The “n+1” Rule for NMR (Don’t Overthink It)
In H-NMR, students get confused by “splitting patterns.”
- The Rule: Look at the neighbor carbon. Count how many hydrogens ($n$) are on it. The peak you are looking at will split into $n+1$.
o Neighbor has 2 H’s? $\rightarrow$ Triplet (2+1).
o Neighbor has 3 H’s? $\rightarrow$ Quartet (3+1).
o Neighbor has 0 H’s? $\rightarrow$ Singlet (0+1).
6. “State Symbol” Hygiene (The Easy Marks)
In Thermodynamics and Kinetics definitions, state symbols are mandatory.
- The Trap: Defining “Enthalpy Change of Formation” without mentioning standard states.
- The Fix: Memorize definitions with the symbols.
o Wrong: “Formation of 1 mole of a compound from its elements.”
o Right: “Formation of 1 mole of a compound from its elements in their standard states under standard conditions.”
The difference between zero marks and full marks often comes down to a single symbol. Compare the ‘lazy’ approach with the ‘hygienic’ approach below:
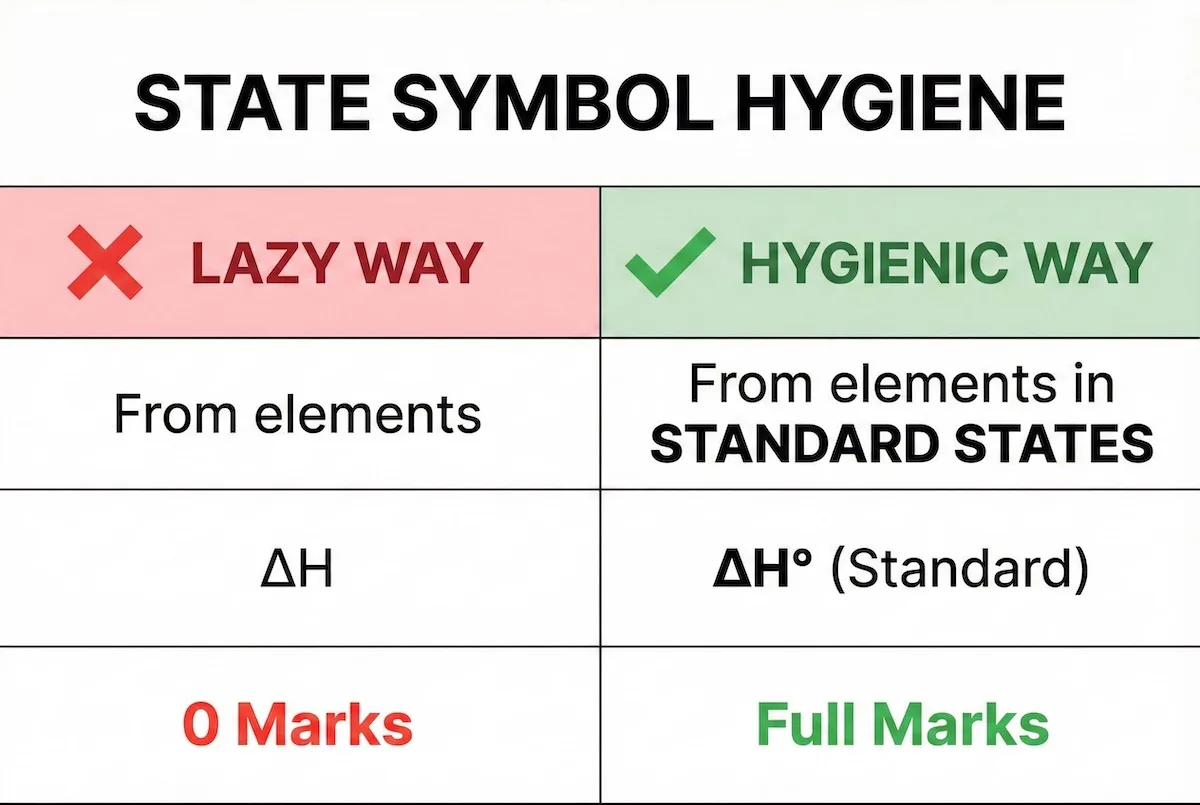
Missing the “standard state” symbol is a guaranteed way to lose easy marks; compare the lazy vs. hygienic approach here.
Always include the standard state symbol (⦵) and specify the state (g/l/s) to guarantee the mark.
7. Mechanism “Curly Arrow” Logic
Don’t just memorize where the arrow goes. Understand why.
- The Rule: A curly arrow always moves from a source of electrons (lone pair or bond) to an electron-deficient atom ($\delta+$).
- The Check: If your arrow starts on a positive charge or an atom without a lone pair, it is wrong.
Read more to get instant, accurate homework help
A common mistake is drawing arrows starting from atoms. To avoid this, visualize the ‘Source to Sink’ rule shown here:
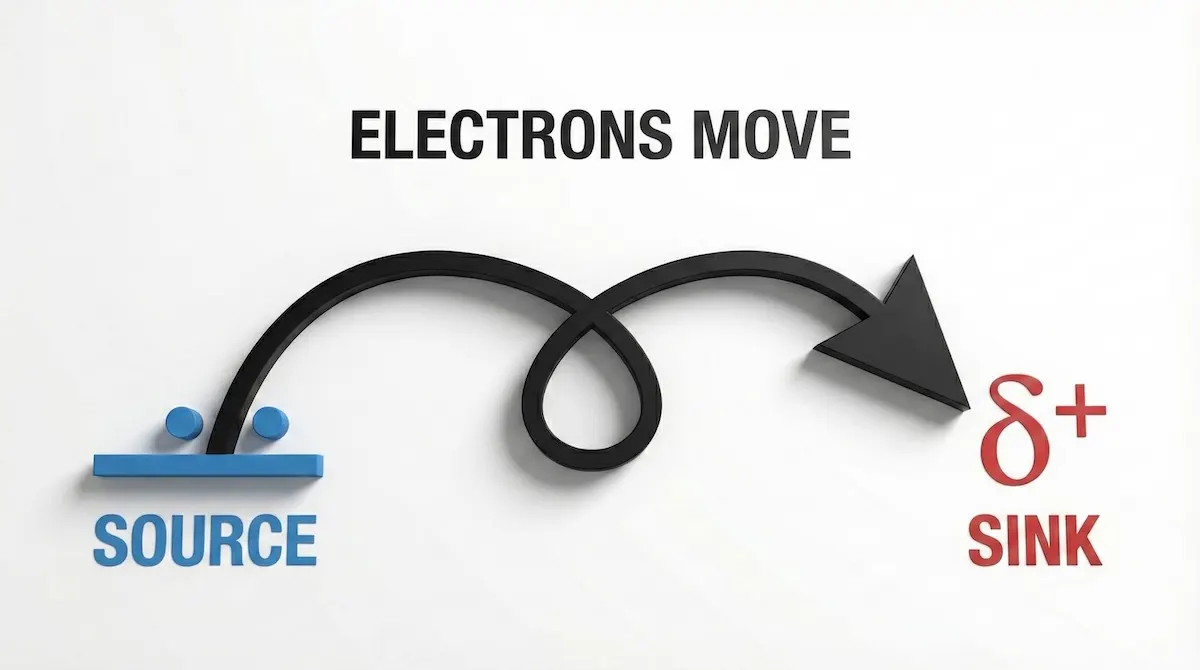
Remember: Curly arrows show the movement of ELECTRONS, not atoms. Always start at the source (lone pair/bond).
If your arrow doesn’t start on a lone pair or a bond (the source), the mechanism is chemically impossible.
Common Mistakes
- Illegible Writing: Writing that looks like one chemical term but is read as another (e.g., $\text{alkene}$ vs $\text{alkane}$) will lose marks (Source 2.3).
- Incorrect Definitions: Using definitions that are close but lack the specific required phrase (e.g., the exact wording for First Ionization Energy) (Source 1.5, 2.3).
- Forgetting Practical Context: Theory papers often test understanding of practical errors, safety, and observation. Do not neglect Paper 3 practical tips (Source 3.4).
Practical Application
Immediately audit your Organic Chemistry knowledge. Pick five key reactions and, using the Blank Page Method, write out the full mechanism and conditions. Then, check the mark scheme for the key words (e.g., curly arrows, nucleophile, intermediate) to ensure your recall is exam-ready.
Key Takeaway
After reading this article, students will be able to replace rote memorization with visual mapping for Organic Chemistry, salvage marks in “failed” practicals, and apply specific mnemonics and error-checks to maximize their score in Cambridge Chemistry 9701.
******************************
This article provides general educational guidance only. It is NOT official exam policy, professional academic advice, or guaranteed results. Always verify information with your school, official exam boards (College Board, Cambridge, IB), or qualified professionals before making decisions. Read Full Policies & Disclaimer , Contact Us To Report An Error

