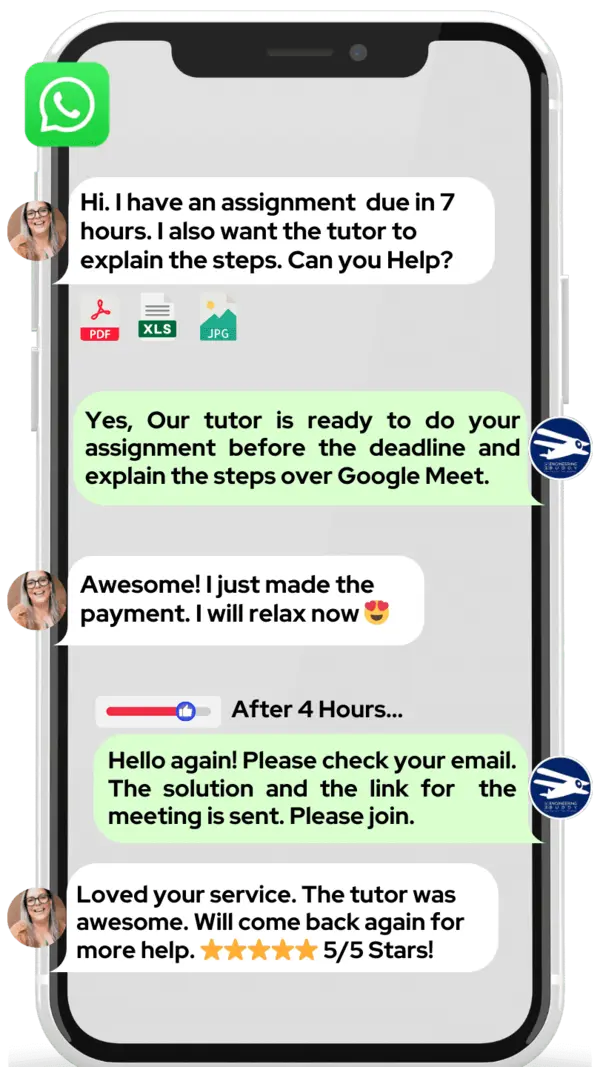
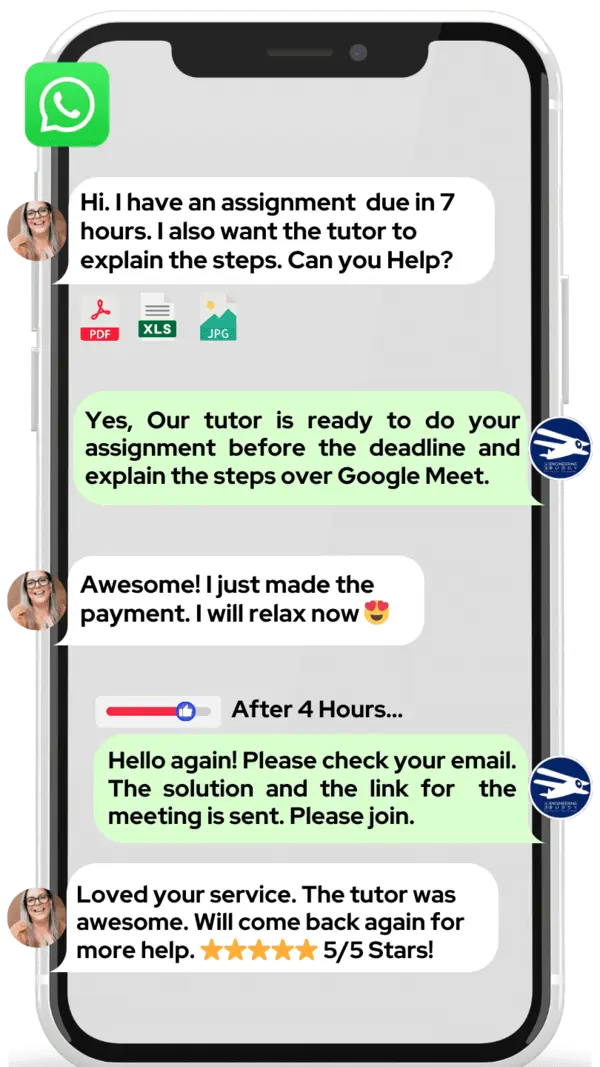
Hire The Best Covalent Bonding Tutor
Top Tutors, Top Grades. Without The Stress!
10,000+ Happy Students From Various Universities
Choose MEB. Choose Peace Of Mind!
How Much For Private 1:1 Tutoring & Hw Help?
Private 1:1 Tutors Cost $20 – 35 per hour* on average. HW Help cost depends mostly on the effort**.
Covalent Bonding Online Tutoring & Homework Help
What is Covalent Bonding?
Covalent bonding arises when two atoms share one or more pairs of electrons to achieve full outer shells. It’s driven by overlap of atomic orbitals, often described by Molecular Orbital (MO) theory. Real‑life: water (H₂O) features two O–H covalent bonds, giving liquid its unique properties.
Popular alternative names: – Electron‑pair bond – Shared‑electron bond – Molecular bond – Electron‑sharing bond – Dative or coordinate covalent bond
Major topics in covalent bonding: - Electron sharing and the octet rule - Bond length, bond energy and bond dissociation energy - Bond polarity, dipole moments and molecular geometry (VSEPR – Valence Shell Electron Pair Repulsion theory) - Resonance structures, formal charge and Lewis structures - Molecular Orbital (MO) theory, including bonding and antibonding orbitals - Applications in organic molecules, drug design and polymer science
In 1916, Gilbert N. Lewis introduced the electron‑pair bond concept, laying groundwork for covalent theory. In 1931 Linus Pauling quantified bond energies and electronegativity differences. Robert S. Mulliken and Friedrich Hund developed Molecular Orbital theory in the early 1930s, merging quantum mechanics with chemical bonding. Erich Hückel’s work on conjugated systems in the 1930s anticipated aromaticity rules. Post‑1950 advances in computational methods like Density Functional Theory (DFT) refined our predictive power. This was a big breakthrough its influence still persists.
How can MEB help you with Covalent Bonding?
If you want to learn about covalent bonding, we at MEB can help. Our online tutoring is one‑on‑one, so you get a tutor all to yourself.
If you are a school, college, or university student and want top grades on assignments, lab reports, tests, projects, essays, or big papers, try our 24/7 covalent bonding homework help. We prefer WhatsApp chat, but if you don’t use it, just email us at meb@myengineeringbuddy.com.
Most of our students come from the USA, Canada, the UK, Gulf countries, Europe, and Australia.
Students ask us for help because: • The subject is hard to learn • They have too many assignments • Questions and concepts take a long time to understand • They have health or personal issues • They work part time • They miss classes or can’t keep up in class
If you are a parent and your ward is struggling with covalent bonding, contact us today. Our tutors will help your ward do well on exams and homework. They will thank you!
MEB also offers help in more than 1000 other subjects. Our tutors and subject experts make learning easier and help students succeed. It’s smart to ask for help when you need it so you can have a less stressful school life.
DISCLAIMER: OUR SERVICES AIM TO PROVIDE PERSONALIZED ACADEMIC GUIDANCE, HELPING STUDENTS UNDERSTAND CONCEPTS AND IMPROVE SKILLS. MATERIALS PROVIDED ARE FOR REFERENCE AND LEARNING PURPOSES ONLY. MISUSING THEM FOR ACADEMIC DISHONESTY OR VIOLATIONS OF INTEGRITY POLICIES IS STRONGLY DISCOURAGED. READ OUR HONOR CODE AND ACADEMIC INTEGRITY POLICY TO CURB DISHONEST BEHAVIOUR.
What is so special about Covalent Bonding?
Covalent bonding is special because it joins atoms by sharing electrons, creating strong links that hold molecules together. This sharing makes covalent compounds stable, with clear-cut shapes and predictable behavior. Unlike ionic or metallic bonds, covalent bonds can form complex structures like DNA or plastics. Such variety lets us build a wide range of materials, from medicines to fragrances.
Covalent compounds often melt and boil at lower temperatures than ionic ones and do not conduct electricity when solid or molten. They are good insulators and tend to be flexible or brittle depending on structure. However, covalent bonds can be harder to dissolve in water and sometimes weaker under certain conditions, limiting their use in harsh or high-temperature environments.
What are the career opportunities in Covalent Bonding?
After mastering covalent bonding, students can move on to organic chemistry, physical chemistry, computational chemistry and materials science. They may join graduate programs or research labs focused on molecular modeling, quantum chemistry or nanotechnology. Recent trends include green chemistry approaches and computer‑aided molecule design.
Career scope covers roles like research chemist, materials scientist, pharmaceutical scientist, nanotechnology engineer and chemical safety officer. Work often involves hands‑on lab experiments, data analysis, computer modeling and quality control. Professionals design new materials, optimize chemical reactions and ensure processes meet safety and quality standards.
We study and prepare for tests on covalent bonding to learn how atoms share electrons and form molecules. A strong grasp of this topic is vital for advanced classes and for competitive exams such as AP Chemistry or university entrance tests. Solid preparation also sharpens problem‑solving skills.
Applications of covalent bonding include drug design, polymer production, battery materials and electronics. Understanding bond strength and molecular shape helps predict reactivity, stability and material properties. Modern methods use computational tools to speed up discoveries and support eco‑friendly practices.
How to learn Covalent Bonding?
Begin by learning what atoms and electrons are. Next, study how atoms share electrons to form covalent bonds. Draw simple Lewis dot structures step by step: write the element symbols, add valence electrons as dots, and pair electrons between atoms. Practice with small molecules like H₂O and CO₂. Review each step until you feel comfortable drawing and naming covalent compounds.
It may seem tricky at first, but covalent bonding isn’t too hard if you follow clear steps and practice regularly. Most students find the basic ideas of shared electron pairs easy once they work through several examples. With each new molecule you draw, your confidence will grow.
You can learn covalent bonding on your own using textbooks and online tools, but some learners benefit from one‑on‑one guidance. A tutor can spot your mistakes, answer your questions in real time, and give you extra practice tailored to your needs. If you find yourself stuck or confused, a tutor speeds up your progress.
Our MEB tutors offer 24/7 live online help and custom practice problems focused on covalent bonding. We guide you through each concept, review your work, and suggest exercises to strengthen weak areas. Whether you need help with homework, exam prep, or deeper understanding, MEB makes tutoring easy, flexible, and budget‑friendly.
Most students spend about 5–10 hours spread over a week to grasp basic covalent bonding concepts and do practice problems. If you dedicate 30–60 minutes per day reviewing lessons and drawing structures, you can feel confident in just a few sessions. More complex topics may take a bit longer, but steady practice brings mastery.
To dive into covalent bonding, check these top resources: YouTube channels include Khan Academy (step‑by‑step tutorials), CrashCourse Chemistry (engaging overviews), Tyler DeWitt (clear explanations), and Bozeman Science (visual demos). Useful websites are KhanAcademy.org for free lessons, Chemguide.co.uk for detailed notes, and LibreTexts.org for interactive texts. Recommended books are Chemistry: The Central Science by Brown et al., Basic Chemistry by Zumdahl, and Chemical Principles by Atkins & Jones. Students also use O.P. Tandon’s Inorganic Chemistry for extra practice.
If you are a college student, parent, or tutor in the USA, Canada, UK, Gulf, etc., and need a helping hand—be it 24/7 online one‑on‑one tutoring or assignment support—our tutors at MEB can help at an affordable fee.








